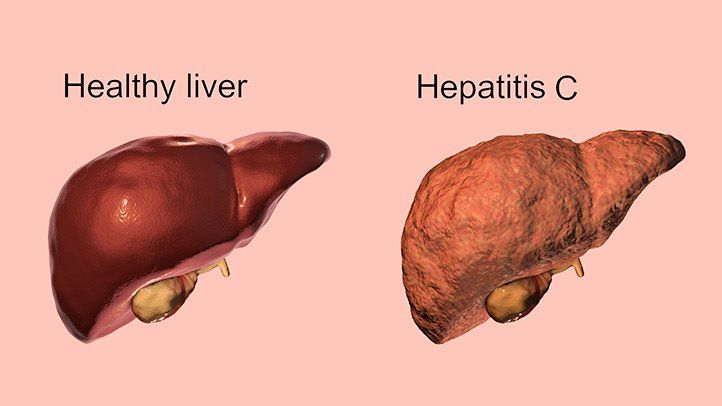
Hepatitis C: Investigations and Diagnosis
The clinical symptoms alone cannot determine the diagnosis of Hepatitis C. The diagnostic test for Hepatitis C involves a study of certain Liver enzymes and Hepatitis C specific antibodies in the blood. Anti Hepatitis C (Anti HCV) virus antibodies can be detected in the blood after 3 to 6 weeks. It may be noted that Anti HCV may not be detected adequately during the acute phase of Hepatitis C. ELISA test for HCV antibody is an important diagnostic test to detect the HCV antibodies, which indicates exposure to the infection.
A. The Qualitative test: (To find our Antibodies to Hepatitis C virus):
- Enzyme immunoassay test (EIA): This test has to be carried out in the initial stage.
- Recombinant Immunoblot Assay (RIBA): This an additional test to support and confirm the diagnosis if the EIA is positive.
Above tests will give a clue if one has an exposure to the infection. However, they cannot indicate the quantum or extent of infection. There are some quantitative tests to determine the amount of virus titer, that is HCV RNA.
B. The Quantitative test:
- The quantitative tests to detect amount (titer) of virus (HCV RNA) are:
It is important to note that the laboratory test as above may be false positive or false negative.
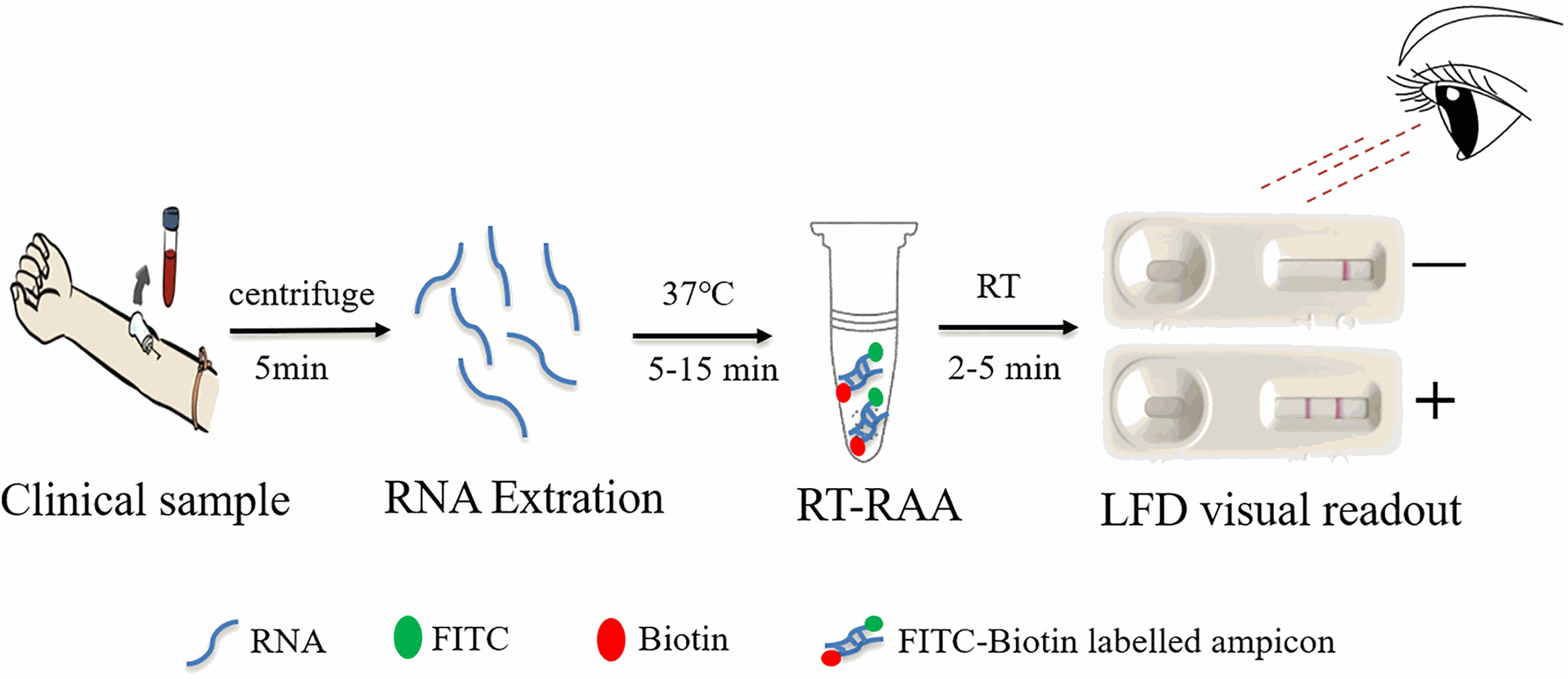
C. Genotype study:
The Hep C virus can have several variants, largely six of them, namely Genotype 1, Genotype 2, Genotype 3, Genotype 4, Genotype 5, and Genotype 6. There are also some subtypes such as 1a, 1b, etc. Patients from different countries have shown different types, which can be briefed as under:
Genotype distribution: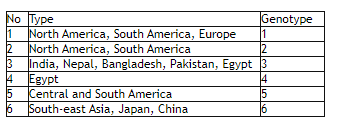
What is Genotyping?
The patents often have this question. Genotyping is nothing but a specific arrangement of the genetic material of RNA in the virus, which has different possibilities, based on which the typing has been done.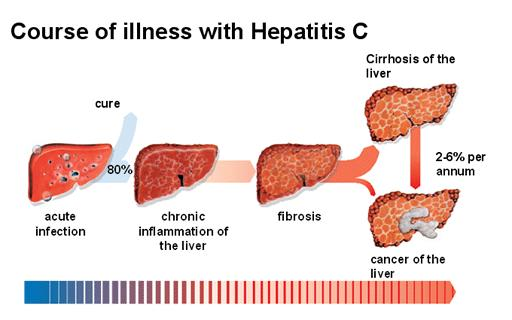
D. Other blood tests:
A complete blood profile is which also consist of the white, red cell and platelet count, hemoglobin count is done. Also, the blood levels of certain liver enzymes such as Alanine Aminotransferase (ALT) and SGOT has to be carried out routinely.
E. Liver biopsy:
Biopsy of liver tissues is not done routinely. It is required to be done to evaluate the scarring (cirrhosis) and cancer of liver. Liver biopsy gives an idea about the extent and severity of fibrosis and cirrhosis.
Anti-HCV ELISA Kit - A First Step in Hepatitis C Diagnosis
The anti-hepatitis C virus antibody (anti-HCV) ELISA kit serves as a crucial tool for initial screening in diagnosing Hepatitis C infection. This enzyme-linked immunosorbent assay (ELISA) leverages the body's immune response to the virus. The kit typically contains pre-coated wells with specific HCV antigens. During the test, a patient's blood serum is added to the wells. If antibodies against HCV are present, they will bind to the immobilized antigens. Subsequent steps involving washing and the addition of an enzyme-linked secondary antibody allow for the visualization of this antigen-antibody complex. A positive result on the anti-HCV ELISA kit from Gentaur indicates potential past or present exposure to HCV. However, it's important to note that a positive result doesn't definitively confirm active infection. A follow-up test that detects the presence of HCV RNA, the virus's genetic material, is necessary to confirm current infection.
Conclusion
Hepatitis C research has yielded significant advancements in understanding the virus, its lifecycle, and the host immune response. The development of highly effective DAAs has revolutionized treatment, offering a cure for the vast majority of infected individuals. However, ongoing research efforts are crucial to address remaining challenges such as optimizing treatment regimens, managing drug resistance, and developing a preventive vaccine. Additionally, improving diagnostic strategies and expanding access to treatment in resource-limited settings remain critical aspects of global Hepatitis C control and eventual elimination.